Tampa General Hospital Is One of Only a Few Sites in the Southeast Participating in Clinical Trial of New Treatment for Normal Pressure Hydrocephalus
Published: Oct 22, 2025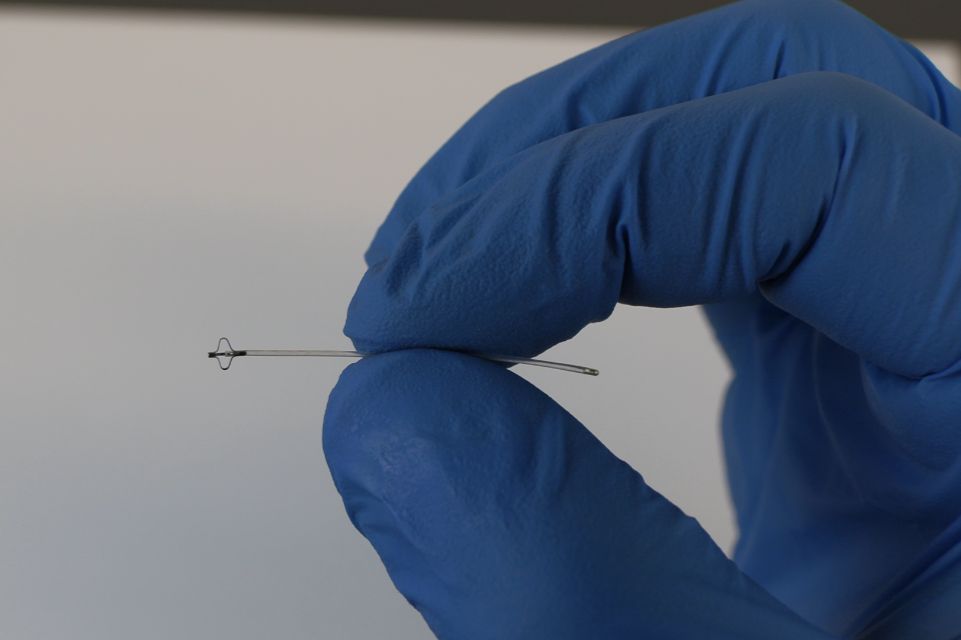
The eShunt System is designed to drain excess cerebrospinal fluid from the brain without the need for open surgery. TGH is one of two sites in Florida selected to participate in the trial.
Tampa, FL (Oct. 22, 2025) – The Neuroscience Institute at Tampa General Hospital (TGH) has opened enrollment in a clinical trial for a new investigational treatment, called the eShunt System, for patients with normal pressure hydrocephalus (NPH). NPH is a progressive, potentially life-threatening condition in which excess cerebrospinal fluid (CSF) builds up in the ventricles of the brain. While NPH affects about 800,000 seniors in the U.S., it is widely underdiagnosed and misdiagnosed, frequently mistaken for Parkinson’s or Alzheimer’s disease, vascular dementia or simply normal aging. It often presents with symptoms such as gait disturbance, urinary incontinence and cognitive impairment.
The prospective multicenter STRIDE Pivotal Study, a randomized controlled trial, is led by CereVasc Inc. and managed locally by the TGH-USF Health Joint Office of Clinical Research. It will evaluate the safety and efficacy of the eShunt System, which is designed to mimic the way the body naturally drains CSF, reducing, and in some cases reversing, NPH symptoms. TGH is one of just a few select trial sites across the nation and one of only two sites in the Southeast offering the treatment.
“The addition of the eShunt system expands our treatment for NPH, offering new hope for patients who previously had limited treatment options,” said Dr. Naomi Abel, a physician with TGH, associate professor of rehabilitation medicine, director of the Adult Hydrocephalus Center in the Department of Neurosurgery, Brain and Spine at the USF Health Morsani College of Medicine. “This marks a major step forward for our research of this condition and how we approach care and also has the potential to improve the quality of life for our NPH patients,” Abel said.
The eShunt System uses a neuroendovascular approach to implant a permanent device at the base of the skull, designed to divert the excess cerebrospinal fluid from the brain into the venous system, while minimizing complications associated with traditional surgical interventions.
“As a leading academic health system in Florida we’re proud to be at the forefront of treating patients with hydrocephalus in our region,” said Dr. Kunal Vakharia, TGH neurosurgeon and associate professor of cerebrovascular/skull base, neurosurgery, brain and spine with the USF Health Morsani College of Medicine’s Department of Neurosurgery, Brain and Spine. “We’re proud to offer this innovative technology that has the potential to transform our standard of care and improve symptoms with minimal downtime. By moving from open surgery to a catheter-based, minimally invasive procedure, we expect better recovery times and improved patient outcomes.”
Additional information can be found here: STRIDE Pivotal Study.
The eShunt Device is an investigational device and has not been approved by the Food and Drug Administration (FDA) or any other regulatory agency for commercial sale. Its safety and effectiveness have not yet been fully established.
This study is funded by CereVasc Inc.
