Tampa General Hospital Performs the First Pediatric Sleep Apnea Implant Surgery in Florida
Published: Nov 1, 2023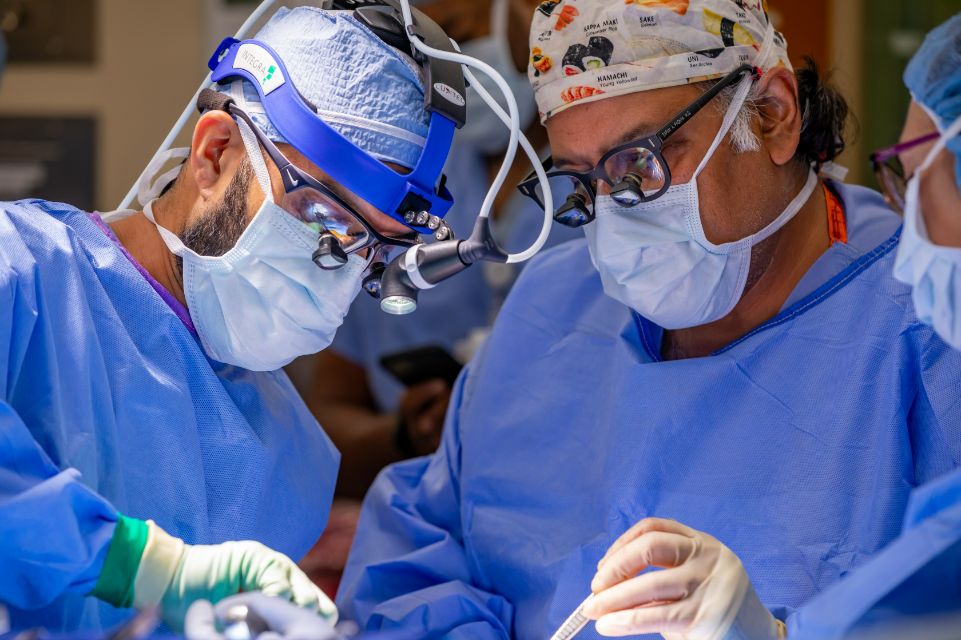
The first use of the implant device reflects the academic health system’s commitment to an integrated, multidisciplinary approach to care that addresses the varied and unique needs
of pediatric patients.
Tampa, FL (Nov. 1, 2023) – Tampa General Hospital (TGH) is announcing a significant milestone for its comprehensive, nationally recognized, TGH Ear, Nose & Throat (ENT) Institute, the successful completion of an Inspire® implant surgery on a pediatric patient diagnosed with Down syndrome and sleep apnea. The groundbreaking procedure is the first of its kind in Florida.
The surgery was performed by University of South Florida (USF) Health otolaryngologist Dr. Abhay Sharma, assistant professor and director of the Division of Interventional Sleep Surgery at the USF Health Morsani College of Medicine. He was assisted by Dr. Tapan Padhya, chief of the TGH ENT Institute, professor, chair and chief, Division of Head and Neck Oncology, professor and chair of the Department of Otolaryngology-Head & Neck Surgery, USF Health Morsani College of Medicine, and founder and co-director of the USF Health ENT Sleep and Snoring Center. Both are full-time academic faculty members in the Department of Otolaryngology-Head & Neck Surgery, USF Health Morsani College of Medicine.
“More than half of children with the genetic disorder Down syndrome have sleep apnea,” Padhya said. “This implantable device marks a substantial improvement in obstructive sleep apnea care for preteens and teenagers with Down syndrome and provides greater peace of mind for their parents.”
“The Inspire implant surgery is minimally invasive and the recovery time for the patient is minimal,” Sharma said. “Before this procedure, traditional treatments for Down syndrome patients with obstructive sleep apnea could include major facial surgery to separate and move a patient’s upper and lower jaws. The implantable device offers a gentler treatment for these young patients.”
The Inspire device is the only implant the U.S. Food and Drug Administration (FDA) has approved to treat sleep apnea and it is one of the few alternatives to using a continuous positive airway pressure (CPAP) machine. The implant is similar to a cardiac pacemaker and is placed under the collarbone during the two- to three-hour surgery. After that, it is operated via a remote control. Electric connectors beneath the skin deliver a small electrical stimulus to the base of the tongue when a patient takes a breath, gently pushing the tongue out to aid airflow. The open airway eliminates the struggle to breathe that characterizes sleep apnea, and patients can experience more restful sleep. To qualify for the pediatric procedure, patients must have Down syndrome. They must be between 12 and 18 years of age, have moderate to severe sleep apnea and be intolerant of a CPAP device. While trials have suggested that a weight below the 95th percentile is safest for children undergoing this procedure, there is no specific weight criteria for children as there is for adults.
The recovery time for an Inspire implant surgery is typically minimal. Following the pediatric procedure at the academic health system, the patient who was 16 years of age at the time of the surgery, remained one night at the TGH Children’s Hospital.
“We are proud to have been a part of this innovative milestone for the TGH ENT Institute,” said Melissa Golombek, vice president of the TGH Children’s Hospital and Women’s Institute. “This is another example of Tampa General’s leading-edge approach to treating all types of complex pediatric medical conditions and is an indicator to the families we serve that our academic health system can provide their children with world-class, advanced care.”
Obstructive sleep apnea is a prevalent condition wherein the upper airway becomes blocked repeatedly during sleep, leading to disrupted airflow due to muscle relaxation around the tongue and throat. This condition affects over 18 million individuals, amplifying the likelihood of heart attack, stroke, high blood pressure and related complications.
According to the National Institutes of Health, 55% to 97% of people with Down syndrome have obstructive sleep apnea. In addition to facial structure differences, excessive weight in patients with Down syndrome often contributes to sleep apnea. It is difficult for young patients with Down syndrome to lose weight and typically these patients can't tolerate CPAP machines due to discomfort and an inability to understand why the machine is necessary and how it works. The implantable device makes it easier for caregivers of patients with Down syndrome to support their needs and improve their quality of life.
“As the indications continue to evolve, we will likely see even younger kids who are having severe sleep apnea who can get this therapy,” Sharma said. “We are optimistic that, with the right protocols, this could be a game-changer for pediatric sleep apnea.”
Sharma added that this procedure could also have major implications for pediatric patients with autism spectrum disorder (ASD), and other neurodevelopmental conditions. Pending approvals, Inspire implant surgery could potentially be performed on any child with sleep apnea.
Florida’s first pediatric sleep apnea implant surgery is the latest leading-edge achievement for the TGH ENT Institute. U.S. News & World Report’s Best Hospitals list for 2023-2024 ranks Tampa General’s ENT program as #2 in Florida and #39 in the United States. In 2014, the TGH ENT Institute was the first to perform an Inspire implant procedure in Florida and since then more than 250 of these surgeries have been completed at Tampa General.
